Partnership Opportunities
As the only event laser-focused on raw materials for cell and gene therapy, the 2nd Raw Materials for Cell & Gene Therapy Summit provided an unmatched platform for suppliers, CDMOs, CROs, and consultants to connect directly with decision-makers shaping the future of CGT manufacturing standards.
Unlike broader conferences, the Summit drilled deep into materials science, regulatory alignment, and supplier strategy, key factors influencing therapeutic success. With the FDA, USP, and leading biotechs like Ultragenyx, Vertex and Abeona in attendance alongside pharma leaders such as Bristol Myers Squibb and Roche, this was where the future of CGT quality took shape and raw materials took center stage, making it a premier partnership opportunity.
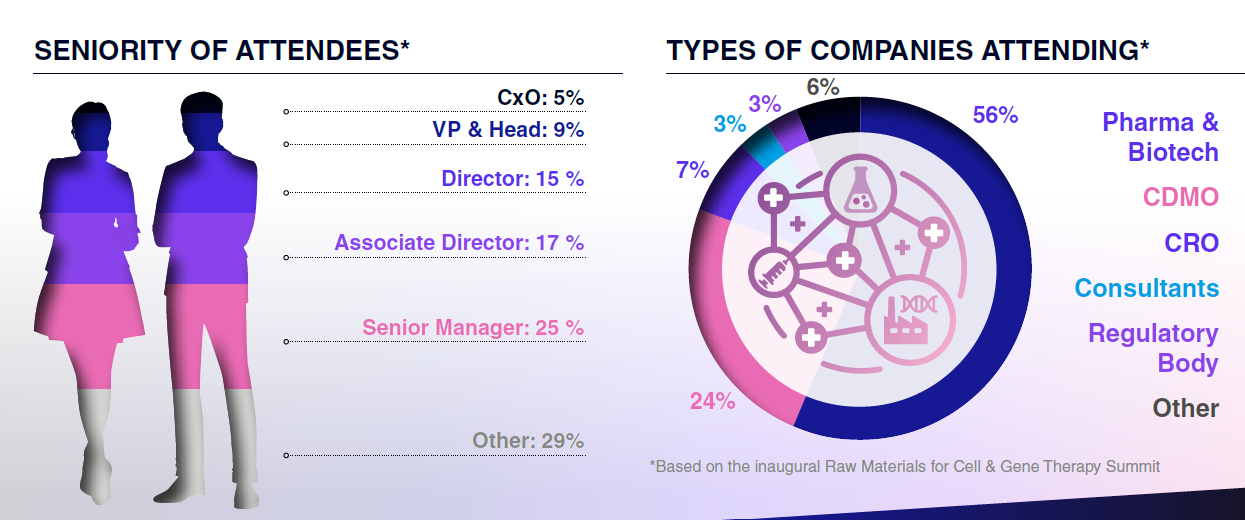
Who Registered in 2025:

Interested in Partnering Next Year? Here's What Biopharma Experts Need:
Single-Use Suppliers:
CGT developers are urgently seeking partners who can deliver high-quality, GMP-ready consumables with traceable, lot-specific data, helping them reduce variability, streamline audits, and accelerate regulatory approval
CDMOs:
Biotech leaders need agile CDMOs that don’t just manufacture - but collaborate. From raw material onboarding to late-stage scale-up, sponsors are looking for CDMOs who can drive speed, flexibility, and compliance across the CGT pipeline
CROs:
With regulatory scrutiny at an all-time high, CGT innovators are turning to CROs who can design and execute cutting-edge material testing strategies, ensuring safety, comparability, and confidence at every clinical stage
Consultants:
Manufacturers are looking for consultants who bring more than advice. They want proven, hands-on strategies to optimize raw material qualification, de-risk supply chains, and navigate complex global regulatory landscapes with clarity and speed
Why Partner with the Summit?
Establish Your Brand as a Raw Materials Thought Leader
Position your organization at the forefront of one of the most complex and rapidly evolving areas in CGT. Through speaking opportunities, panel involvement, and curated roundtables, you’ll elevate your visibility as a trusted partner in raw material innovation and compliance
Drive Qualified Pipeline Growth & Partnerships
This Summit is engineered for results, bringing together buyers and influencers with real purchasing power. Whether you're a supplier, CDMO, CRO, or consultant, you'll gain access to decision-makers ready to collaborate, co-develop, and source smarter for next-gen therapies
Access Focused Cell & Gene Therapy Raw Materials Audiences
Engage directly with decision-makers in MSAT, Quality, Supply Chain, and Regulatory Affairs who are actively shaping raw material strategies for clinical and commercial CGT pipelines. This is your opportunity to showcase solutions to biopharma stakeholders tasked with vendor qualification, testing, and risk mitigation
Who Will You Meet?
Interested in partnering in 2026?
Get in touch to benefit from market intelligence, showcase your world-class solutions and forge valuable commercial collaborations.
Explore Related Events:
Hanson Wade is committed to bringing the cell and gene therapy spaces bespoke conferences catering for every niche, with the aim of getting better drugs to patients faster.
Take a look at our related events below to find out more about our tailored conferences, designed to put you in the room with decision makers from both leading and emerging companies in these growing spaces

Having trouble downloading the prospectus? Let us know here and we'll email it to you instead.
